Electrolysis
Electrolysis is the process of splitting up a liquid with
electricity into its components. This process needs a liquid that conducts
electricity - an electrolyte. Electrolytes have free ions that move around in
them at random. Salts like NaCl, when dissolved in water forms an effective
electrolyte, that can easily been used to perform electrolysis with ease.
In short, any substance that has free ions is an
electrolyte. Molten ionic substances have free ions and therefore form
electrolysis. However, the high temperature involved in the process has to be
taken into account, when using them.
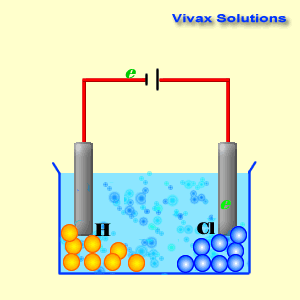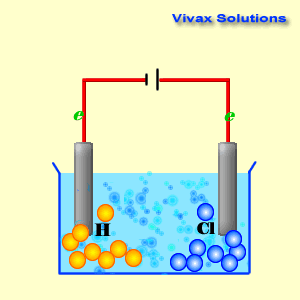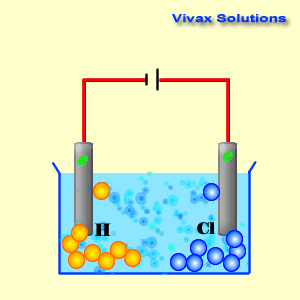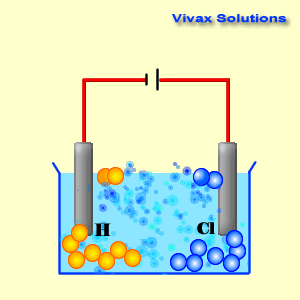
In electrolysis, a battery is connected to two 'rods' that
goes into the electrolyte.
They are called electrodes - electrode connected to
the negative terminal is called cathode and the one that is connected to the
positive terminal is called anode.
A cathode is a source of electrons; it has a collection of
them. The positive ions which are hungry for electrons, naturally, approach the
cathode to get them. However, if there are different types of positive ions,
then a competition breaks out in reaching the cathode - the kind of things that
happen at sales, when announced suddenly. It is now a matter of survival of the
most active. The most active positive ions get electrons and become their
respective atoms: if it is a metal, it deposit itself on the cathode; if it is a
gas, they just bubble out from the cathode.
At anode, the negative ions undergo similar scenario. In the
end, most active negative ions abandon their excess electrons and become their
respective atoms. If it is a gas, it bubbles out from the anode and if it is a
metal, it gets deposited on the anode.
The animation is using
NaCl as the electrolyte. The ions in it are H+ , Na+ ,
Cl- and OH- . However, the main players are H+
and Cl- , as they are the stronger ions that dominate the
electrolysis process.
As you can see, the hydrogen ions approach the cathode and
obtain electrons and become hydrogen gas. At the anode, on the other hand,
chloride ions give up their electrons and become chlorine gas. Both sodium ions
and hydroxyl ions have been prevented from active participation by the dominance
of hydrogen and chloride ions.
In the end, the NaCl solution breaks up into hydrogen and
chlorine. This is electrolysis.